Introduction
AI is transforming drug discovery, turning a traditionally slow and costly process into one that’s faster, smarter, and more precise. By analyzing vast datasets and simulating biological interactions, AI helps scientists identify promising compounds and reduce failure rates early. This shift is not only accelerating time-to-market but also reshaping the economics of pharmaceutical R&D.
What is AI and Why Does It Matter in Drug Discovery?
Definition of AI and Its Core Technologies
Artificial Intelligence refers to computer systems capable of performing tasks that typically require human intelligence, such as recognizing patterns, interpreting language, and making decisions. In the context of drug discovery, AI uses algorithms and models to analyze vast datasets from genomics, chemistry, and clinical trials. This allows researchers to uncover hidden relationships, accelerate hypotheses, and reduce time spent on manual experimentation.
Core AI technologies used in drug discovery include machine learning, deep learning, and natural language processing. These tools enable prediction of molecular behavior, identification of drug targets, and extraction of insights from scientific literature. By integrating these capabilities, AI empowers pharmaceutical companies to innovate faster, cut development costs, and improve the chances of clinical success.
The Growing Role of AI in Transforming Drug Discovery
AI is becoming a vital force in drug discovery, driving innovation across the entire pipeline. It accelerates research, improves decision-making, and reveals insights hidden in vast scientific datasets. With growing access to data and computing power, its impact on pharma continues to grow.
A key benefit is the reduction in time and cost. AI can screen millions of compounds or identify drug targets in days instead of months. This speed boosts efficiency and makes it easier to pursue treatments for rare or complex conditions.
AI also enhances accuracy and reduces risk. It learns from biological systems and past clinical failures to guide smarter decisions. The result is a faster, more reliable path from lab to patient.
Explore how AI’s evolution over the past decade has reshaped drug discovery timelines, costs, and clinical success in our retrospective guide on AI in drug discovery.
Key Statistics and Trends Highlighting AI Adoption in Drug Discovery
The global AI in drug discovery market was valued at $0.9 billion in 2023 and is projected to reach $4.9 billion by 2028, growing at a 40.2% CAGR. North America holds the largest share, accounting for over 57% of the market in 2023.
AI is drastically reducing drug discovery timelines. A landmark case demonstrated that novel compound design and validation could be achieved in just 46 days, a process that typically takes over a year.
AI is also driving breakthroughs in complex drug classes. MIT researchers used AI to discover the antibiotic Halicin in only three days, later validated in vivo. These results highlight AI’s role in accelerating discovery while improving accuracy and success rates.
Business Benefits of AI in Drug Discovery
AI is not just accelerating drug discovery, it’s reshaping the economics of the entire industry. From faster development to smarter investments, the business impact is both measurable and strategic.
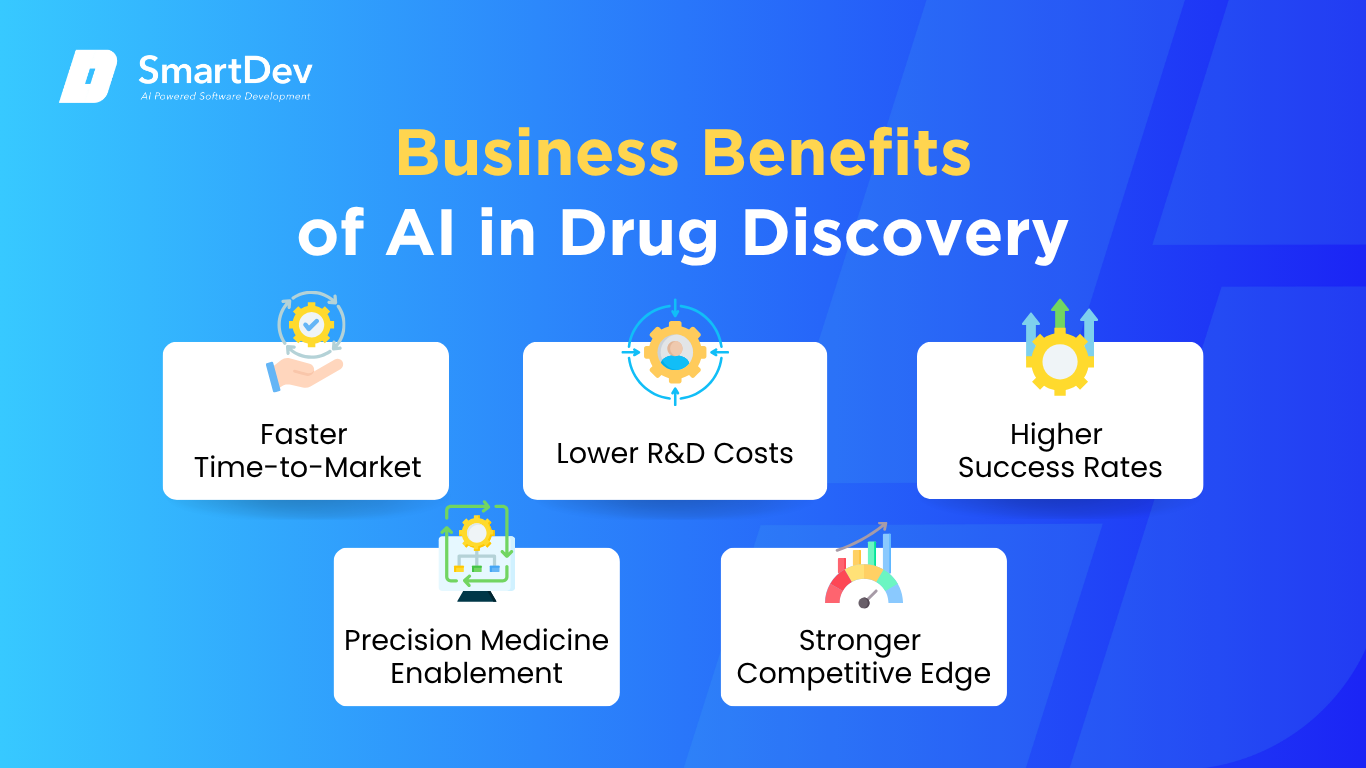
1. Faster Time-to-Market
AI streamlines early-stage drug discovery through automation and predictive analytics. Tasks like target identification, compound screening, and lead optimization that used to take months can now be completed in a matter of days. This speed allows companies to push candidates into clinical trials faster than ever before.
Reaching the market sooner translates to earlier revenue, extended exclusivity periods, and competitive advantage. It’s especially critical in fast-moving therapeutic areas and during public health emergencies. In an industry where every month counts, acceleration is a clear strategic win.
2. Lower R&D Costs
Drug development can exceed $2.6 billion per approved compound, with most of the cost concentrated in the early research phases. AI reduces the need for brute-force screening and extensive manual labor by simulating compound behavior virtually. It also helps predict failures early, avoiding costly late-stage trial collapses.
This efficiency allows both large pharma and lean biotech startups to operate with more agility and lower burn rates. More experiments can be run at a fraction of the cost. That means greater innovation with less financial risk.
3. Higher Success Rates
AI improves decision-making by analyzing multi-dimensional datasets, from genomics to clinical outcomes, to flag risks and validate targets. It can identify toxicity, poor absorption, and off-target effects early in the pipeline. This dramatically reduces attrition rates in clinical trials.
Better candidate selection means fewer failures and higher return on R&D investment. Investors are more confident backing pipelines built on data-driven insights. Companies can prioritize what works, reducing waste and increasing overall productivity.
4. Precision Medicine Enablement
AI enables companies to design therapies for narrowly defined patient populations based on biomarkers and genetics. This leads to treatments that are more effective, with fewer side effects. It’s a cornerstone of precision medicine and critical for tackling complex or rare diseases.
From a business perspective, this opens up high-margin niche markets and strengthens partnerships with healthcare providers. Personalized therapies also support value-based care models where payment is tied to outcomes. This boosts both pricing power and real-world impact.
5. Stronger Competitive Edge
Companies that leverage AI position themselves as innovation leaders. This differentiation attracts talent, funding, and strategic partnerships with other biotech, pharma, or tech firms. It also enhances the company’s brand as forward-thinking and tech-enabled.
In a crowded and highly regulated industry, having advanced AI capabilities is a long-term moat. It improves resilience and adaptability to scientific and market changes. Being ahead on the AI curve isn’t just smart, it’s a competitive necessity.
Challenges Facing AI Adoption in Drug Discovery
Despite its potential, AI in drug discovery comes with real barriers that can’t be ignored. From data quality to regulatory complexity, these challenges must be addressed to unlock full value.
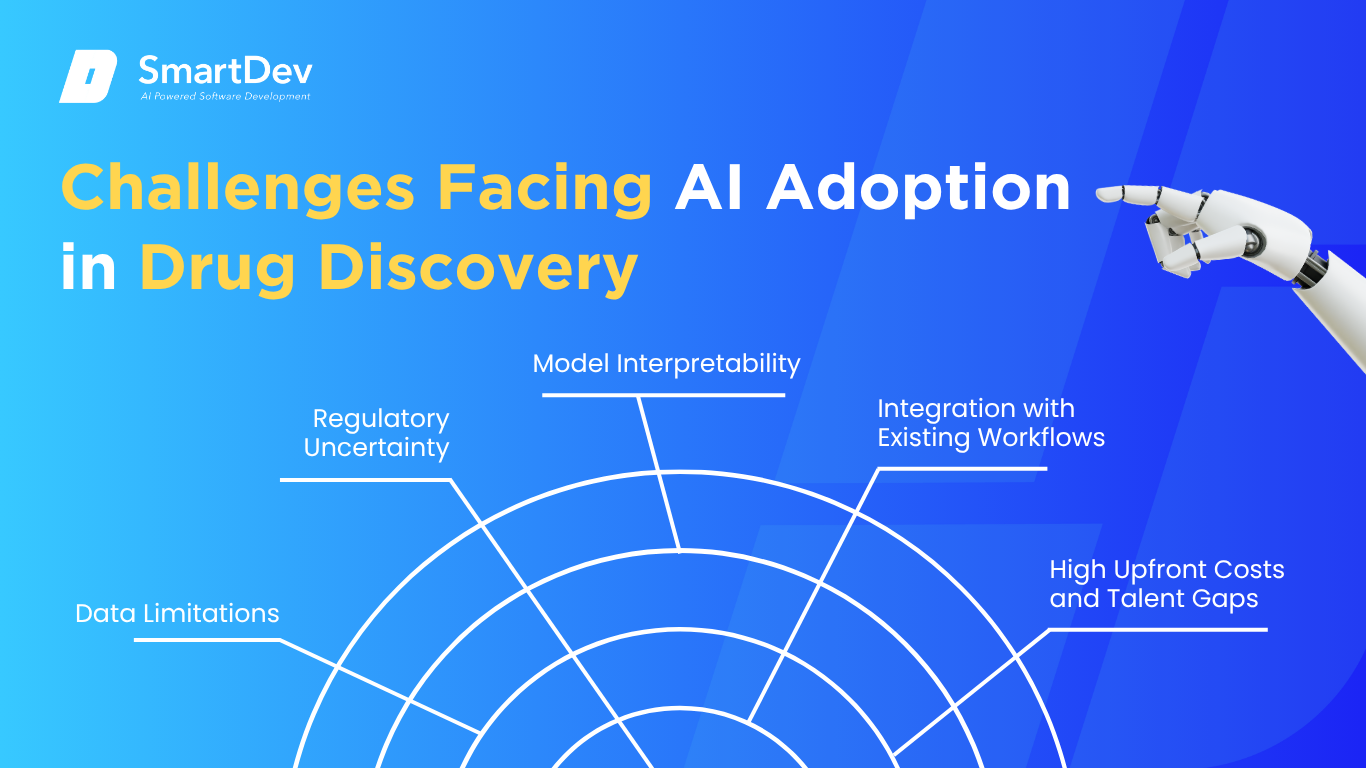
1. Data Limitations
AI models rely heavily on large volumes of high-quality, well-labeled biomedical data. In drug discovery, datasets are often siloed, incomplete, or inconsistent, making training difficult. Poor input leads to unreliable predictions, which can derail early-stage research.
Many pharma companies still lack robust data infrastructure and standards. Integrating data from multiple sources – omics, clinical, chemical – is complex and expensive. Without clean, standardized datasets, even the most advanced AI models fall short.
2. Regulatory Uncertainty
AI-driven drug discovery raises novel regulatory questions, especially when models are used to make safety or efficacy predictions. Current frameworks from the FDA and EMA were not built with AI in mind, leading to gray areas. Approval timelines and requirements remain inconsistent.
This lack of clarity slows down innovation and increases compliance risk. Companies may hesitate to fully embrace AI tools without clear regulatory pathways. More guidance is needed to balance innovation with patient safety.
Understand how to align AI innovation with evolving data privacy standards in our security-focused guide.
3. Model Interpretability
Many AI models, especially deep learning architectures, are black boxes, offering predictions without clear explanations. In a highly regulated, life-critical industry like pharma, this lack of transparency is a major barrier. Scientists and regulators need to understand why a model made a decision.
Trust in AI depends on its ability to provide not just results, but rationales. Without explainability, adoption will be limited to low-stakes decisions. Explainable AI (XAI) tools are emerging but not yet widely adopted in drug discovery.
Explore how ethical and explainability concerns are addressed in real-world deployments in our detailed guide on AI ethics concerns.
4. Integration with Existing Workflows
Implementing AI isn’t just about the tech, it’s about transforming how teams work. Many research and clinical workflows are built on legacy systems and manual processes that don’t integrate easily with AI tools. The result is friction, redundancy, or underuse of AI’s capabilities.
Successful integration requires cross-functional collaboration between data scientists, chemists, and clinicians. It also demands cultural shifts and reskilling, which take time and leadership buy-in. Without these, AI investments often underperform.
5. High Upfront Costs and Talent Gaps
Building and deploying AI solutions in drug discovery requires significant upfront investment. This includes not just infrastructure and tools, but highly specialized talent in bioinformatics, AI, and systems biology. Such expertise is in short supply and high demand.
Smaller biotech firms often struggle to afford or attract the talent needed to deploy AI effectively. Outsourcing can help, but it adds dependency and potential IP risks. The talent and cost barriers can slow AI adoption across much of the industry.
Specific Applications of AI in Drug Discovery
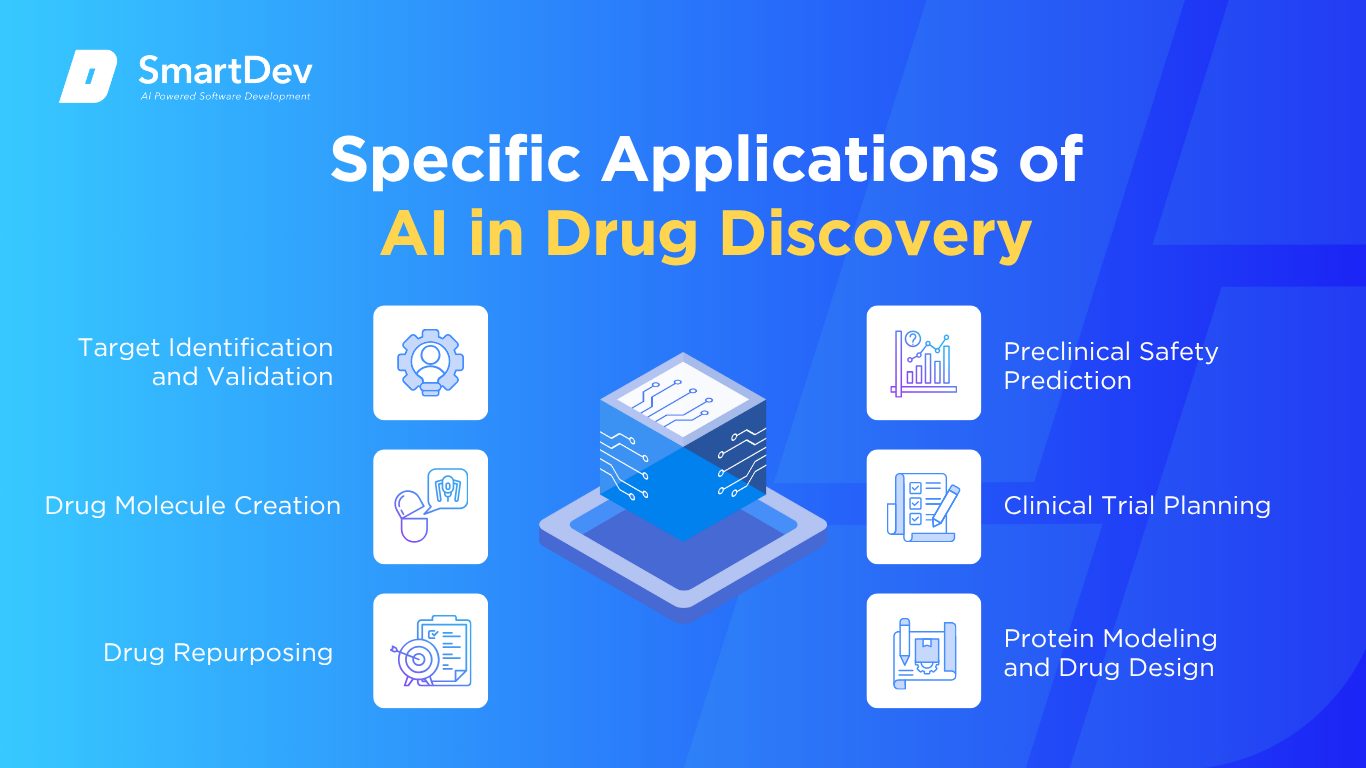
1. Target Identification and Validation
AI is revolutionizing target identification by analyzing complex biological datasets such as genomics, proteomics, and transcriptomics. These datasets are processed using machine learning algorithms to find disease-associated biomarkers and therapeutic targets. This helps uncover relationships that may not be immediately evident through traditional research.
Natural language processing and knowledge graphs further enhance this capability by synthesizing information from published studies and databases. This integration supports the generation of comprehensive target profiles. AI models can then rank targets based on their druggability and relevance to disease pathways.
The outcome is a more streamlined approach to prioritizing and validating targets. It significantly cuts down on time and resources spent in early-stage research. However, the reliability of results hinges on the diversity and quality of the input data.
Real-world example:
Every Cure developed the MATRIX AI platform to map thousands of disease-drug relationships. It identified sirolimus as a treatment for Castleman disease, leading to the remission of co-founder Dr. David Fajgenbaum. MATRIX continues to evaluate over 3,000 FDA-approved drugs for potential new applications across 10,000 diseases.
Discover how AI transforms vast unstructured datasets into actionable insights in our guide on unstructured AI.
2. Drug Molecule Creation
AI has enabled the generation of novel molecular structures with desirable pharmacological properties through de novo drug design. Generative models, including GANs and reinforcement learning systems, rapidly explore vast chemical spaces to propose new compounds. These molecules are optimized for potency, bioavailability, and synthetic feasibility.
Structure prediction tools such as AlphaFold contribute by providing detailed models of protein targets. This allows AI systems to simulate interactions between candidate molecules and their intended targets. Integration with synthesis planning tools accelerates the movement from design to lab testing.
Overall, this approach shortens drug discovery timelines and opens possibilities for targeting previously undruggable proteins. It also reduces reliance on traditional trial-and-error methods. Rigorous experimental validation remains a necessary step to confirm computational predictions.
Real-world example:
Insilico Medicine used Chemistry42 and PandaOmics to design a CDK20 inhibitor for idiopathic pulmonary fibrosis. This AI-generated compound progressed from ideation to Phase I trials in under 30 months, compared to the industry average of 5–6 years. The program was among the first AI-designed drugs to enter human testing.
3. Drug Repurposing
AI facilitates drug repurposing by uncovering new therapeutic applications for existing compounds. This process involves mining clinical records, biomedical literature, and chemical databases to detect hidden connections. Machine learning models assess similarities in disease mechanisms and drug action.
Techniques such as natural language processing extract actionable insights from unstructured text, while knowledge graphs map interactions between molecules and biological systems. These insights help prioritize compounds with strong evidence for alternate indications. AI models can generate hypotheses that are then tested through experimental or clinical validation.
Repurposing helps reduce development time and risk by leveraging existing safety and efficacy data. It is particularly valuable during health crises that require rapid therapeutic responses. Transparency in prediction rationale is essential to support regulatory acceptance.
Real-world example:
In early 2020, BenevolentAI’s AI models identified baricitinib as a potential treatment for COVID-19. The drug, initially developed for rheumatoid arthritis, was found to inhibit SARS-CoV-2 entry via JAK1/2 inhibition. It received emergency use authorization and was included in WHO treatment guidelines.
4. Preclinical Safety Prediction
AI plays a critical role in evaluating the safety profile of drug candidates during preclinical stages. Models trained on historical assay data predict ADMET properties, identifying compounds likely to fail due to toxicity or poor bioavailability. This early insight saves resources and minimizes risk in later stages.
Techniques like QSAR modeling and deep learning identify structural alerts and forecast off-target effects. AI can also simulate how drugs interact with metabolic enzymes and organ systems. These tools provide a more comprehensive view of a compound’s risk profile.
The strategic benefit is a more efficient screening process and higher success rates in preclinical testing. However, outcomes depend heavily on the quality and representativeness of training datasets. Regulatory bodies increasingly scrutinize the validity of in silico models in decision-making.
Real-world example:
Researchers at Sumitomo Dainippon Pharma developed DSP-0038, a novel 5-HT1A partial agonist, using AI-guided toxicity modeling. The compound advanced to Phase I trials in just 12 months – considerably faster than the typical 4–6 year cycle. This rapid progression underscores the value of in silico safety profiling.
5. Clinical Trial Planning
AI enhances clinical trial planning by improving the selection of suitable patients and optimizing trial protocols. Algorithms analyze electronic medical records, genomics, and prior trial data to identify ideal candidate groups. This supports better stratification and predictive modeling of trial outcomes.
Natural language processing helps extract relevant information from clinical notes and documentation. Predictive models simulate enrollment timelines, adherence patterns, and potential safety issues. This allows sponsors to proactively adjust designs to improve success rates.
As a result, trials become faster, more efficient, and cost-effective. It also increases the likelihood of regulatory approval by ensuring robust data collection. Still, AI models must be interpretable and aligned with data privacy standards.
Real-world example:
Pfizer employs AI via partnerships with Tempus and Saama Technologies to enhance clinical operations. Their systems analyze real-world data to match patients to trials and anticipate FDA feedback. These tools helped optimize trial submissions and expedite COVID-19 vaccine development.
6. Protein Modeling and Drug Design
Advances in AI have transformed protein modeling, a foundational aspect of rational drug design. Tools like AlphaFold predict protein structures at atomic resolution, enabling researchers to model target interactions more accurately. This improves the design of molecules with high binding specificity.
AI also models protein-ligand and protein-protein interactions, expanding drug discovery to more complex targets. These predictions can be integrated with generative tools to suggest new chemical entities for synthesis. Such integrations streamline early discovery workflows.
This capability allows pharmaceutical teams to address previously inaccessible targets. However, experimental validation is still required to confirm model predictions. Regulators demand high standards for accepting AI-generated structural insights.
Real-world example:
Isomorphic Labs, a DeepMind spinout, partners with Novartis and Eli Lilly to develop AI-designed therapies using AlphaFold 3. These efforts have generated multiple preclinical candidates targeting difficult proteins. The company expects to enter human trials in 2025.
Need Expert Help Turning Ideas Into Scalable Products?
Partner with SmartDev to accelerate your software development journey — from MVPs to enterprise systems.
Book a free consultation with our tech experts today.
Let’s Build TogetherExamples of AI in Drug Discovery
Real-world case studies highlight how AI is actively transforming the drug discovery pipeline. These success stories show how AI is being applied by leading innovators to solve critical challenges and accelerate results.
Real-World Case Studies

1. BenevolentAI: Drug Repurposing During the COVID-19 Crisis
BenevolentAI leveraged its proprietary AI platform to analyze biomedical data and literature at scale, aiming to find treatment candidates for COVID-19. In early 2020, it identified baricitinib, a JAK inhibitor used for rheumatoid arthritis, as a potential option to reduce virus entry into cells. This discovery led to clinical trials, and baricitinib was later granted Emergency Use Authorization by the FDA and endorsed by the WHO for use in hospitalized COVID-19 patients.
The AI platform helped streamline hypothesis generation by processing millions of scientific publications and molecular interaction data in days. BenevolentAI’s approach demonstrated the real-world speed advantage AI offers in urgent therapeutic discovery. The case became a landmark example of AI successfully guiding drug repurposing for a global health emergency.
2. Insilico Medicine: AI-Discovered Drug Enters Human Trials
Insilico Medicine created a novel small molecule inhibitor targeting fibrosis using its AI platforms PandaOmics and Chemistry42. The molecule, ISM001-055, moved from initial discovery to Phase I human clinical trials in under 30 months – a process that typically takes over five years. The compound showed promising results in preclinical validation and is currently in further clinical testing.
This project marked the first instance where every stage of drug development – from target discovery to molecular design – was handled by AI systems. It demonstrated how end-to-end AI integration can drastically cut costs and timelines. The project received recognition for its speed and potential, setting a new benchmark for AI-powered biotech innovation.
3. DeepMind: Structural Biology Revolution with AlphaFold
In 2020, DeepMind released AlphaFold 2, an AI system that predicts protein structures with atomic-level accuracy. This innovation solved a 50-year-old challenge in biology and was hailed by the journal Nature as one of the biggest scientific breakthroughs of the decade. Its public release enabled researchers to access over 200 million protein structures via the AlphaFold Protein Structure Database.
AlphaFold is now used widely by pharmaceutical companies for structure-based drug discovery, helping design drugs for diseases with limited structural data. Its successor, AlphaFold 3, further models protein-ligand interactions, enabling deeper applications in drug design. These tools are revolutionizing target validation and ligand docking in drug discovery pipelines worldwide.
Innovative AI Solutions
AI technologies are evolving rapidly, enabling new methods for simulating, designing, and validating drugs. Multi-agent frameworks and autonomous pipelines are increasingly used to model entire drug development workflows, allowing faster iteration and task coordination. These solutions enhance efficiency by mimicking decision-making roles traditionally carried out by multidisciplinary teams.
Recent advances also extend beyond structure prediction into dynamic modeling of molecular interactions. AI now supports predictions of how drugs bind to proteins, nucleic acids, and other targets with unprecedented accuracy. These developments are reshaping how early-stage research is conducted in both academic and industrial labs.
AI-Driven Innovations Transforming Drug Discovery
Emerging Technologies in AI for Drug Discovery
Generative AI is revolutionizing drug discovery by creating novel molecules optimized for specific biological targets. These AI models learn from vast chemical and biological datasets to generate compounds with desired properties like solubility, efficacy, and safety. As a result, drug design timelines are being reduced significantly, enabling faster progression from concept to viable candidates.
Computer vision is also transforming how researchers analyze cellular and molecular data during early-stage screening. By interpreting complex images of biological samples, AI can detect subtle changes and patterns that human eyes might miss. This leads to more accurate assessments of how compounds affect biological systems, improving decision-making in the earliest phases of drug development.
AI’s Role in Sustainability Efforts
AI is playing a critical role in making drug discovery more sustainable by reducing unnecessary experiments and resource use. Through predictive analytics, AI can identify promising compounds earlier and eliminate low-potential candidates before expensive lab testing begins. This targeted approach minimizes material waste and shortens development cycles, conserving both time and resources.
In addition, AI contributes to energy efficiency by optimizing computational workloads and experimental processes. Cloud-based platforms and automated workflows reduce the need for energy-intensive lab operations. These advancements help pharmaceutical companies lower their environmental impact while maintaining high research productivity.
How to Implement AI in Drug Discovery
Implementing AI in drug discovery requires a structured, strategic approach. The following steps outline how to integrate AI seamlessly to enhance research efficiency and outcomes.
Step 1: Assessing Readiness for AI Adoption
Before bringing AI into your drug discovery pipeline, evaluate your current capabilities and digital maturity. Look at where manual processes still dominate, such as compound screening, data analysis, or target identification, as these are often ideal for automation. Starting with well-defined, high-impact areas makes it easier to measure progress and build momentum.
Leadership alignment is just as crucial as technical readiness. Successful AI adoption often requires rethinking traditional research workflows and encouraging cross-functional collaboration. Without strong support from decision-makers, even promising pilots can lose direction or fail to scale.
Discover how technical leaders drive AI readiness and adoption strategies in our guide for tech leads.
Step 2: Building a Strong Data Foundation
AI tools are only as good as the data they rely on, so clean, well-organized information is essential. Begin by consolidating your experimental results, chemical databases, and assay records into a central system that’s accessible and secure. Structured data helps algorithms uncover meaningful patterns faster and with greater accuracy.
Make sure your organization invests in good data governance practices. This includes establishing consistent formats, verifying data quality, and ensuring compliance with regulatory standards. With a trusted data backbone in place, AI can deliver insights that drive better scientific decisions.
Explore why clean, well-governed data is the foundation of successful AI adoption in our data management guide.
Step 3: Choosing the Right Tools and Vendors
Selecting the right AI solution starts with defining your scientific and operational goals. Whether you’re aiming to streamline compound screening or improve clinical predictions, the tool you choose should align with your existing workflows. Avoid overcomplicating things, focus on platforms that integrate easily and provide transparency in how they process your data.
Equally important is finding a partner who understands the complexities of life sciences. Look for vendors that offer domain expertise, scientific validation, and responsive support. When your AI provider is a strategic collaborator – not just a tech vendor – it becomes much easier to deliver long-term value.
Step 4: Pilot Testing and Scaling Up
Before a full rollout, test AI in a controlled, high-impact area of your R&D process. A pilot focused on early-stage candidate selection, for instance, can help you assess performance without major disruption. These experiments create proof points and provide practical learnings that guide broader adoption.
Use what you learn to refine your AI strategy and gain internal support. Pay close attention to model outputs, team feedback, and measurable improvements in efficiency or accuracy. Once a pilot proves successful, you can confidently expand to other areas with greater scale.
Step 5: Training Teams for Successful Implementation
For AI to thrive in your organization, teams need more than just access, they need understanding. Offer training that explains not only how the technology works, but also how it complements existing expertise. This builds trust and helps teams see AI as a tool that enhances, not replaces, their role.
Encourage ongoing collaboration between scientists, data analysts, and technical teams. AI adoption is most effective when insights flow across departments and feedback loops stay active. With the right training and culture, your people become AI enablers rather than roadblocks.
Measuring the ROI of AI in Drug Discovery
Key Metrics to Track Success
Understanding the return on investment from AI begins with tracking the right metrics. Improvements in productivity, such as reduced screening times or faster compound optimization, are clear indicators that AI is creating value. Many organizations also report higher throughput in early discovery, allowing researchers to explore more drug candidates in less time.
Cost savings are another critical factor in evaluating AI’s impact. AI can lower expenses by reducing the need for repetitive wet-lab experiments and streamlining trial design. When machine learning accurately predicts which compounds will fail early, teams avoid costly setbacks in later phases of development.
Case Studies Demonstrating ROI
AstraZeneca partnered with CSPC Pharmaceuticals in a $5.2 billion deal to co-develop preclinical candidates using AI, including $110 million upfront and milestone-based incentives. The collaboration highlights AI’s potential to reduce early-stage costs and accelerate candidate selection, making large-scale investment more efficient and less risky.
Janssen used machine learning to forecast COVID-19 trial site performance, cutting enrollment time by 33% and reducing participant needs by 25%. These improvements translated into measurable cost savings and faster time-to-market for their vaccine development efforts.
Common Pitfalls and How to Avoid Them
One of the most common mistakes in AI adoption is underestimating the importance of data quality. When teams feed inconsistent or unstructured data into models, they risk generating misleading outputs that slow progress instead of speeding it up. Organizations should invest early in data governance to ensure accuracy and consistency from the start.
Another challenge is misaligned expectations around AI’s capabilities. While AI is powerful, it’s not a silver bullet, and unrealistic goals can lead to frustration or stalled initiatives. Teams that treat AI as a partner in decision-making, rather than a replacement, are more likely to see sustainable, long-term returns.
Learn how to evaluate AI model effectiveness and ROI with our practical guide on AI performance metrics.
Future Trends of AI in Drug Discovery

Predictions for the Next Decade
AI is set to evolve from a supportive tool into a central force in autonomous drug design. Future systems will simulate entire discovery pipelines, integrating generative models, biological simulations, and real-time lab data to create closed-loop drug development workflows. This transformation will significantly reduce the time and cost of bringing new therapies to market.
Another key trend is the convergence of AI with personalized medicine. Advances in genomics, multi-omics data, and patient stratification will allow AI to design treatments tailored to individual profiles. Businesses that adapt early to these technologies will be better positioned to lead in precision therapeutics and deliver more effective, targeted care.
How Businesses Can Stay Ahead of the Curve
To stay competitive in the AI-driven future of drug discovery, companies must invest in continuous learning and cross-functional collaboration. This includes upskilling scientific teams in data science fundamentals and embedding AI expertise within R&D departments. Organizations that foster a culture of experimentation and agile decision-making will be better equipped to adapt quickly.
Equally important is forming strategic partnerships with AI-native startups or academic innovators. Staying informed on emerging technologies and piloting them early allows businesses to iterate faster and scale what works. Being proactive rather than reactive ensures a stronger position as the industry evolves.
Conclusion
Key Takeaways
AI is reshaping drug discovery by accelerating timelines, improving accuracy, and reducing costs across key stages. From molecule design to trial optimization, it enables faster, data-driven decisions that boost R&D efficiency.
Success depends on clean data, strategic implementation, and a workforce ready to collaborate with technology. Businesses that start small, scale smart, and invest in AI readiness will gain a long-term edge in the evolving landscape of drug development.
Moving Forward: A Strategic Approach to AI-Driven Transformation
As AI becomes a cornerstone of modern drug discovery, now is the moment to rethink your R&D strategy for speed, precision, and scalability. From accelerating molecule design to improving trial outcomes and cutting development costs, AI is no longer just an innovation, it’s a competitive necessity.
At SmartDev, we help biotech and pharmaceutical organizations integrate AI solutions that deliver real-world results. Whether you’re exploring early-stage pilots or scaling a full AI-driven pipeline, our team guides you through each step—from data readiness to deployment.
Explore our AI-powered software development services to see how we support businesses discovery innovation with custom tools, predictive models, and actionable insights.
Contact us today to learn how AI can transform your discovery process and position your organization at the forefront of pharmaceutical innovation.
—
References:
- Artificial Intelligence In Drug Discovery Market Report, 2030 | Grand View Research
- Generative AI in Drug Discovery Market Size, Share, and Trends 2024 to 2034 | Precedence Research
- AI designed, synthesised and validated new drug in 46 days | Drug Target Review
- Can A.I. Find Cures for Untreatable Diseases—Using Drugs We Already Have? | The New Yorker
- From Start to Phase 1 in 30 Months: AI-discovered and AI-designed Anti-fibrotic Drug Enters Phase I Clinical Trial | Insilico Medicine
- Pfizer Announces Positive Topline Results From Phase 3 Study of Hemophilia A Gene Therapy Candidate | Pfizer
- AlphaFold: a solution to a 50-year-old grand challenge in biology | Google DeepMind
- ‘It will change everything’: DeepMind’s AI makes gigantic leap in solving protein structures | Nature
- PharmAgents: Building a Virtual Pharma with Large Language Model Agents | arXiv
- AstraZeneca signs AI research deal with China’s CSPC for chronic diseases | Reuters
- AI Accelerates Drug Discovery and Clinical Trials, Reducing Time and Costs | MedPath






