Introduction
The pharmaceutical industry stands at a crossroads. With escalating R&D expenditures, lengthier drug development pipelines, and ever-intensifying regulatory scrutiny, you are likely feeling the pressure to innovate faster, safer, and more cost-effectively. Artificial Intelligence (AI) is not just a technological trend; it is a transformative enabler that can help you address these very challenges head-on. From accelerating molecule discovery to enhancing supply chain integrity, AI is beginning to redefine what is possible in modern pharmaceutical operations.
This comprehensive guide is designed to help you understand exactly how AI is delivering tangible results in your field. We will explore practical use cases, the business value AI offers, the obstacles you might face, and strategic insights to help your team stay competitive in this evolving landscape.
What is AI and Why Does It Matter in Pharma?
-
Definition of AI and Its Core Technologies
Artificial Intelligence (AI) refers to the simulation of human intelligence by machines that are capable of performing tasks such as problem-solving, reasoning, learning, and language understanding. According to IBM, core components of AI include machine learning (ML), natural language processing (NLP), and computer vision. These technologies enable machines to detect patterns, draw conclusions from vast datasets, and continuously improve over time without explicit programming. Learn more about AI here.
Within the pharmaceutical industry, AI represents more than automation; it becomes a vital co-pilot in navigating complex research, development, and operational challenges. Whether your goal is to discover new drug compounds, enhance patient safety, or refine regulatory processes, AI offers tools that are not only scalable but also capable of learning from historical and real-time data.
-
The Growing Role of AI in Transforming Pharma
AI is rapidly becoming integral to how pharmaceutical companies operate, and its influence is expanding across all functions of the value chain. In drug discovery, AI algorithms are now used to identify promising compounds far more quickly than traditional lab experiments. These platforms simulate millions of molecular interactions to predict which combinations are most likely to succeed, significantly cutting the time and financial investment needed during the preclinical phase.
In the area of clinical development, AI transforms your clinical trials by optimizing everything from patient recruitment to safety monitoring. Using AI, you can tap into real-world data from electronic health records and genomic databases to identify candidates more precisely and reduce dropout rates. This not only shortens trial timelines but also increases your likelihood of regulatory approval.
From an operational perspective, AI is proving its value in manufacturing and distribution. Predictive maintenance tools monitor equipment to preemptively resolve issues, avoiding downtime and ensuring compliance with strict quality standards. AI-enabled supply chain analytics help you anticipate demand changes and reduce waste, which is especially crucial for temperature-sensitive or high-cost medications.
-
Key Statistics or Trends in AI Adoption
The shift toward AI in pharmaceuticals is not just theoretical; it is backed by measurable trends. According to McKinsey’s 2024 report, over 70 percent of pharmaceutical executives say their organizations have made significant investments in AI applications, particularly in drug discovery and clinical trial optimization. Companies are recognizing the ability of AI to streamline development timelines and enhance research accuracy.
Another compelling data point comes from Deloitte’s 2023 analysis, which found that AI-powered patient recruitment platforms reduced trial startup times by 20 to 50 percent. This is a game-changer when you consider the traditional delays caused by low enrollment and site selection challenges. Your team can now leverage AI to identify and enroll diverse patient populations more efficiently.
Furthermore, the global AI in healthcare market is experiencing explosive growth. Grand View Research projects that the market will grow from 10 billion USD in 2023 to more than 30 billion USD by 2030, driven largely by pharmaceutical applications. This trend reinforces the urgency to integrate AI into your business model if you intend to stay ahead in a highly competitive environment.
Business Benefits of AI in Pharmaceutical Industry
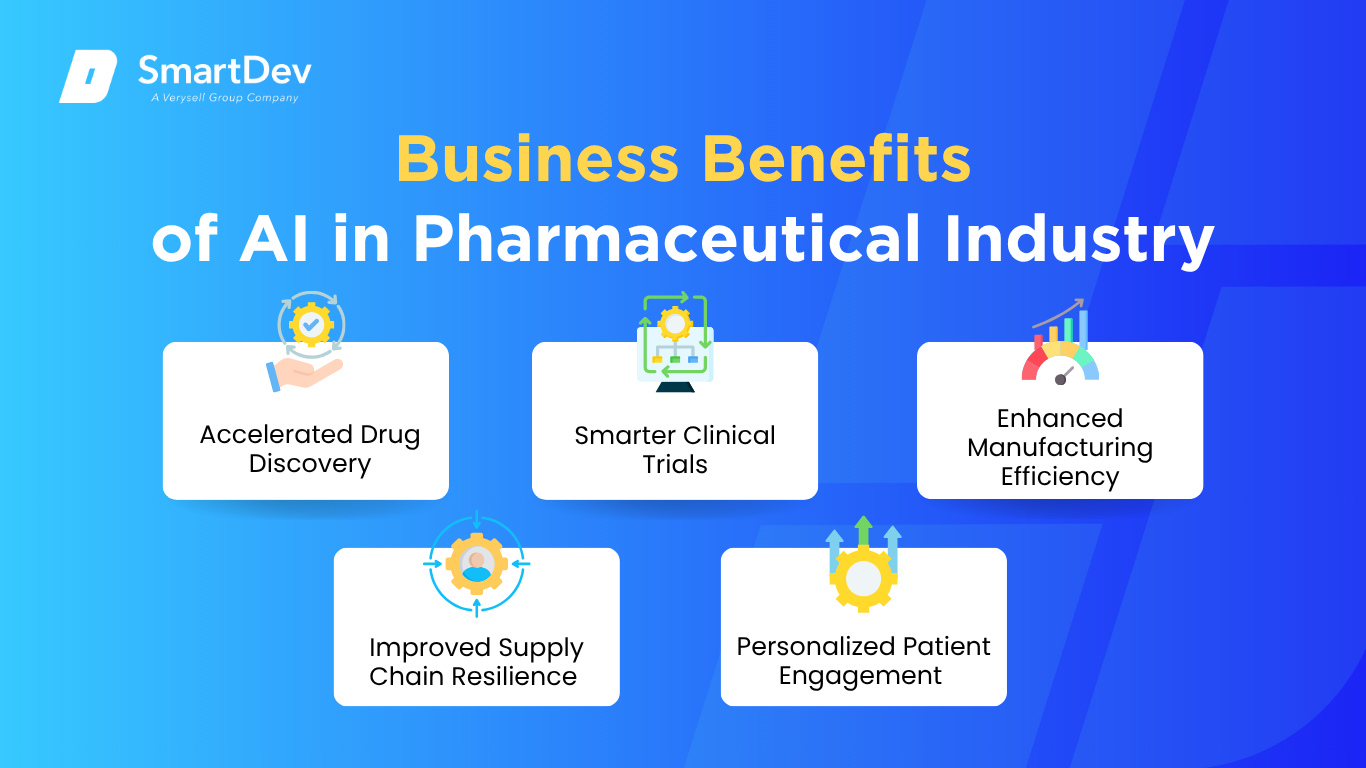
The business impact of AI in the pharmaceutical industry is no longer speculative. It is now clear that AI delivers meaningful improvements to productivity, cost-efficiency, and innovation. Below are five key areas where AI can directly benefit your business operations.
-
Accelerated Drug Discovery
One of the most resource-intensive stages of pharmaceutical development is drug discovery. Traditional methods can take years and involve extensive trial and error. With AI, you gain access to algorithms that can simulate and evaluate millions of molecular combinations in silico. This allows your research teams to zero in on the most promising compounds and eliminate less viable options early on.
By adopting AI-driven discovery platforms, you reduce not only time to target identification but also the financial risk associated with failed experiments. AI enables you to explore a wider chemical space than ever before, uncovering novel drug candidates for diseases that have previously been difficult to target.
-
Smarter Clinical Trials
Conducting clinical trials is both costly and time-consuming, but AI can help you navigate this complexity more effectively. Natural language processing tools analyze patient data to ensure optimal inclusion criteria, while predictive algorithms can identify those at risk of dropping out of the study. This ensures a more stable and compliant trial cohort.
Moreover, AI facilitates adaptive trial designs that allow real-time protocol adjustments based on interim results. This level of agility helps you maintain regulatory compliance while improving trial outcomes. Ultimately, your organization can accelerate time to market without compromising on safety or scientific integrity.
-
Enhanced Manufacturing Efficiency
Manufacturing pharmaceutical products involves stringent regulatory oversight, making efficiency both critical and challenging. AI-powered systems monitor your production lines in real time, identifying bottlenecks and anomalies that might compromise quality. Predictive maintenance models forecast equipment failures before they happen, preventing costly disruptions.
Furthermore, computer vision applications are used for quality control by inspecting each unit with precision that far exceeds human capabilities. These technologies ensure that your products meet compliance standards while reducing rework and waste. This leads to higher throughput and more consistent output quality.
-
Improved Supply Chain Resilience
In an era where supply chain disruptions can lead to severe consequences for patient care, AI offers a solution grounded in predictive analytics. AI models analyze historical trends, market fluctuations, and even environmental data to forecast demand more accurately. This helps you plan inventory levels, streamline procurement, and mitigate risks of overstock or shortage.
Real-time tracking systems powered by AI also offer visibility into transportation routes, flagging delays or risks such as temperature deviations in cold-chain logistics. These insights enable your team to make proactive decisions that safeguard product integrity and ensure timely delivery.
-
Personalized Patient Engagement
As the pharmaceutical industry becomes more patient-centric, AI is enabling you to engage patients more meaningfully and effectively. Virtual health assistants powered by natural language processing can provide patients with personalized medication guidance, adherence reminders, and answers to frequently asked questions.
These systems not only improve patient outcomes but also generate real-world evidence that feeds back into your R&D and pharmacovigilance programs. By capturing structured data from patient interactions, AI helps you understand how your medications perform outside the clinical trial setting, thereby informing future innovations.
Challenges Facing AI Adoption in Pharma
While the potential of AI in the pharmaceutical industry is vast, several obstacles can hinder successful adoption. Understanding these challenges is crucial for developing a realistic and sustainable AI strategy.

-
Fragmented and Non-Standardized Data
Your data is likely spread across multiple platforms and stored in varying formats, from lab notebooks to enterprise systems. This fragmentation makes it difficult for AI algorithms to access and process information effectively. The lack of standardized data structures further compounds the problem, reducing the accuracy and reliability of AI-driven insights.
To unlock the full potential of AI, your organization must prioritize data governance and integration. This includes investing in centralized data lakes, adopting common data standards, and fostering collaboration between departments to maintain data quality.
And the important thing is the way you secure the utilize of AI in your pharmaceutical ones, so read more about AI and Data Privacy: Balancing Innovation with Security
-
Regulatory Uncertainty
Regulatory bodies such as the FDA and EMA are still in the process of defining guidelines around AI applications in pharmaceuticals. This uncertainty can make it challenging for you to incorporate AI into regulated processes like clinical trials or product labeling.
To navigate this evolving landscape, you should involve regulatory experts early in the AI implementation process. Engage in dialogue with regulators, participate in pilot programs, and ensure thorough documentation to demonstrate transparency and compliance.
-
Model Interpretability and Transparency
Many AI models operate as black boxes, delivering outputs without clearly explaining how decisions were made. This lack of interpretability can be a serious issue in a field as sensitive as healthcare. When AI suggests a dosage adjustment or flags a trial participant for exclusion, you need to understand the rationale.
Investing in explainable AI (XAI) tools allows you to meet both internal accountability and external regulatory requirements. By ensuring transparency, you increase trust in AI systems among clinicians, regulators, and patients alike.
-
Integration with Legacy Systems
The pharmaceutical sector often relies on legacy IT infrastructure that was not designed for AI integration. These systems can become bottlenecks when attempting to deploy AI solutions that require real-time data access and interoperability.
Your organization may need to modernize parts of your tech stack or implement middleware that bridges the gap between new AI tools and existing systems. This transition requires close coordination between IT, data science, and operational teams.
-
Ethics, Bias, and Data Privacy
AI models are only as good as the data they are trained on. If your training data lacks diversity, the AI system may produce biased outcomes that fail to account for variations across different populations. This is a significant concern in drug development and patient engagement.
Moreover, you must ensure that all AI initiatives comply with data privacy regulations such as GDPR and HIPAA. Implementing robust de-identification protocols, consent mechanisms, and secure data storage is not optional; it is essential for building trustworthy AI solutions.
Before investing in AI systems in your pharma institution, the awareness of ethical problems is important, you can read more at AI Ethics Concerns: A Business-Oriented Guide to Responsible AI
Specific Applications of AI in the Pharmaceutical Industry
-
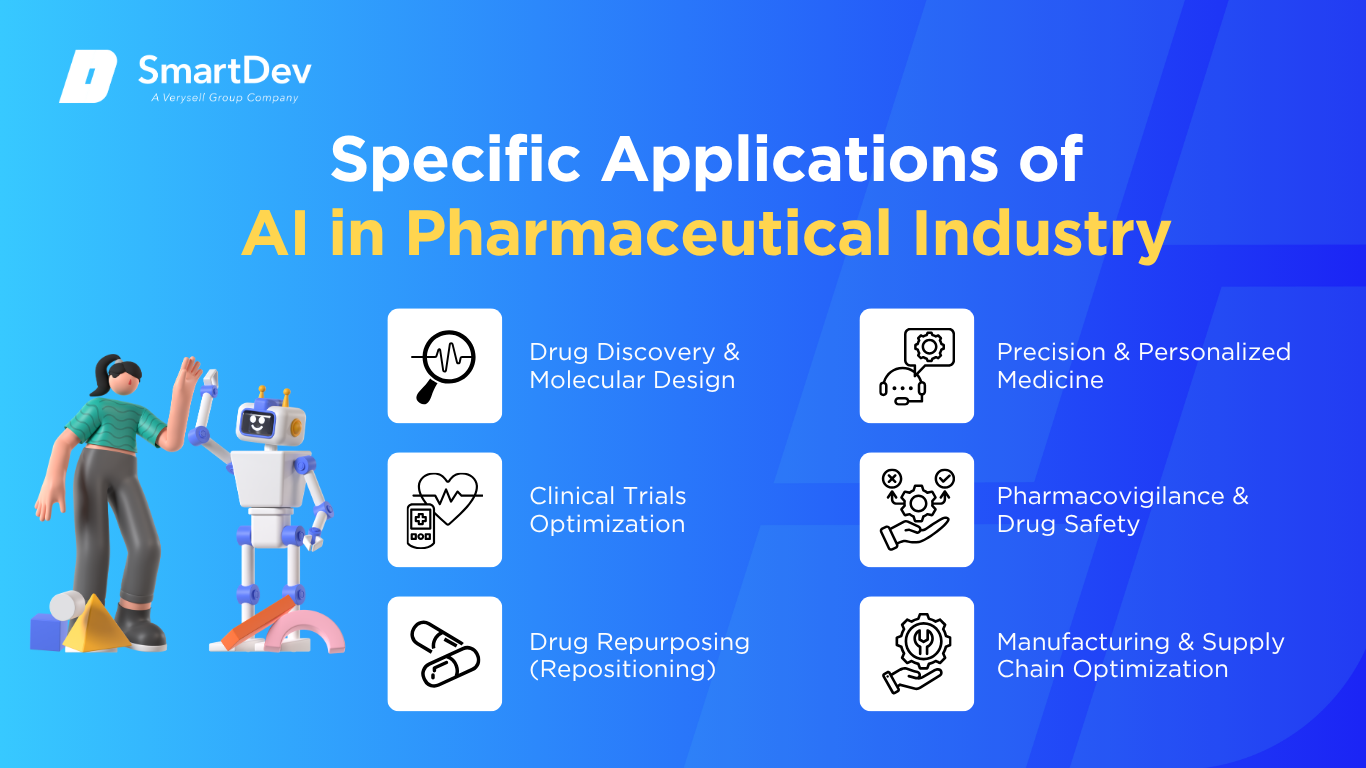
Drug Discovery & Molecular Design
AI-based drug discovery uses deep learning and generative models to identify and design novel molecules that target specific diseases—solving the problem of lengthy, costly R&D cycles. These systems process large chemical and biological datasets, simulate molecular interactions, and integrate with lab workflows to generate candidates with optimized efficacy/toxicity profiles.
By accelerating hypothesis testing and reducing attrition rates, they deliver strategic value through faster time-to-market and lower costs. Technical considerations include model interpretability, quality of training data, and ensuring reproducibility.
Real‑World Example:
Insilico Medicine applied GANs and reinforcement learning to design a molecular candidate, entering human trials in 2023 and reducing discovery time and cost by nearly 10× versus traditional pipelines.
-
Clinical Trials Optimization
AI improves patient recruitment, site selection, protocol design, and real-time monitoring using predictive analytics and natural language processing on EHRs and trial databases. These systems model trial outcomes, identify candidates faster, and adapt protocols dynamically to enhance retention and reduce delays. This optimizes trial timelines, lowers costs, and improves success probabilities. Ethical issues include data privacy (e.g., compliance with GDPR), algorithmic bias, and transparency in decision-making.
Real‑World Example:
Parexel piloted an AI model that auto-generates safety reports 30–45 minutes faster than manual methods and cuts trial costs/timelines by up to 80 %.
-
Drug Repurposing (Repositioning)
Computational drug repurposing employs machine learning to analyze molecular, clinical, and real-world data to discover new indications for existing drugs—helping avoid high costs and time associated with de novo drug development. These systems use EHRs, literature mining, and molecular screening to match drugs to new therapeutic contexts effectively. This brings value in strategic pipeline expansion and risk mitigation. It’s critical to validate predictions clinically and manage intellectual property and regulatory hurdles.
Real‑World Example:
The Exscalate4Cov project used computational repurposing to flag raloxifene as a candidate for early-stage COVID-19, illustrating rapid pandemic response potential.
-
Precision & Personalized Medicine
AI-driven precision medicine leverages genomic, proteomic, imaging, and clinical data to stratify patients and craft tailored treatments. These systems use machine learning to predict drug response based on biomarkers and individual profiles, integrating into clinical decision support workflows. The strategic value lies in enhanced efficacy, safety, and improved patient outcomes. Limitations include potential algorithmic bias and ensuring equitable model performance across diverse populations.
Real‑World Example:
Exscientia’s AI system matched AML therapy to patients based on tumor profiles, resulting in a cohort where 54 % maintained disease control nearly 33 % longer than the previous regimen.
-
Pharmacovigilance & Drug Safety
Machine learning systems automate detection and prediction of adverse drug reactions (ADRs) by analyzing EHRs, social media, and pharmacological databases. AI methods (e.g., LSTM, knowledge graphs) monitor large-scale drug safety data in real time and flag safety signals earlier. This enhances patient impact by reducing harm and improving regulatory compliance, while challenges revolve around data quality, false positives, and interpretation.
Real‑World Example:
Roche implemented AI tools combining real-world datasets and genomics to monitor ADRs in real time, improving detection accuracy and response speed.
-
Manufacturing & Supply Chain Optimization
AI-driven predictive maintenance uses sensors and machine learning to forecast equipment failures in biologics plants—preventing production delays. AI also enhances supply chain forecasting, demand planning, and quality control processes. Operational value includes reduced downtime, lower waste and costs, and improved production throughput. Data integration, model transferability across plants, and cybersecurity are core operational considerations.
Real‑World Example:
A global pharma company using C3 AI Reliability predicted equipment failure up to 10 days in advance, reducing false positives by 90 % and capturing $150 million in annual benefits per plant.
Whether you’re a CTO or CEO aiming to drive innovation in pharma, or a researcher seeking faster, more accurate outcomes, now is the time to act. Explore cutting-edge AI solutions at AI Solution Delivery to integrate tools like drug discovery algorithms, clinical trial optimization, and pharmacovigilance automation into your operations.
Examples of AI in the Pharmaceutical Industry
AI’s real-world deployments prove its transformative power. Below are case studies that illustrate measurable impact across functions and operations.
Real‑World Case Studies

Case Study 1: Exscientia – AI‑Driven Drug Design
Exscientia is a biotechnology company dedicated to leveraging AI to accelerate the drug design process. Their platform enables rapid generation and optimization of novel molecular compounds.
Traditional drug discovery workflows are time consuming, with high failure rates during early stages. Researchers often confront inefficiencies due to manual screening and unpredictable candidate performance. These manual processes also limit the potential to explore vast chemical spaces efficiently.
Exscientia implemented an AI-driven platform that integrates deep learning and reinforcement learning. The platform systematically generates and optimizes molecular structures against defined disease targets. This approach replaces repetitive human-led iteration with automated, intelligence-guided exploration.
The system uses neural network architectures to model complex protein–ligand interactions. Reinforcement learning frameworks reward molecules that demonstrate desired properties, such as potency and low toxicity. Models train on curated biochemical datasets and iteratively propose improved compounds.
Exscientia successfully advanced multiple compounds to early-phase clinical trials. The company reports a substantial reduction in drug development time, with early-stage candidate identification achieved in under two years. They also documented a measurable decrease in R&D expenditures per candidate.
By employing a structured AI approach, Exscientia demonstrates that artificial intelligence can redefine traditional drug discovery. The firm’s success illustrates that intelligent algorithmic systems can both accelerate timelines and improve molecular quality, creating a strong foundation for future pharmaceutical innovation.
Case Study 2: Insitro with Eli Lilly & BMS – Biological Data Modeling
Insitro is a technology-driven biopharma company that specializes in biological data modeling to identify therapeutic targets. In collaboration with Eli Lilly and Bristol Myers Squibb, they endeavor to elevate internal R&D pipelines.
Complex biological processes often generate high-dimensional data that is difficult to interpret using conventional analytics. Traditional methods frequently fail to capture non-linear patterns in genomics and proteomics. As a result, identifying reliable drug targets remains a persistent challenge.
Insitro developed a machine learning pipeline that can extract predictive insights from multi-omic datasets. The platform harmonizes data across genomics, transcriptomics, and cellular imaging. It incorporates rigorous statistical and computational rigor to discern actionable biological signals.
Deep neural networks are trained on labeled biological profiles in both healthy and disease states. The model identifies biomarker signatures and predicts therapeutic responsiveness. Methods include convolutional and autoencoder-based models to process complex, multidimensional inputs.
Collaborations with Eli Lilly and BMS yielded faster and more reliable target identification. The AI system improved pipeline prioritization accuracy and reduced time to candidate selection. Internal assessments show increased confidence in hypothesis quality before entering experimental validation.
Insitro’s integration of machine learning in biological data analysis exemplifies a balanced, data-centric path toward accelerating drug discovery. By transforming complex datasets into predictive insights, they provide pharmaceutical partners with enhanced clarity and strategic decision-making.
Case Study 3: Roche – Real‑Time Pharmacovigilance
Roche implemented an advanced pharmacovigilance system designed to detect adverse events more rapidly and accurately. The system processes both structured and unstructured real-world data.
Traditional pharmacovigilance monitoring relies heavily on manual review of reports and logs. Detection is often delayed due to data latency and reporting fragmentation. As a result, timely identification of adverse events remains a significant safety challenge.
Roche introduced a machine learning engine that continuously scans electronic health records, social media, and scientific publications. The platform normalizes disparate data sources and applies natural language processing alongside predictive modeling. This innovation enables proactive monitoring and early safety signal detection.
Next mining algorithms extract event-related information from unstructured text. Time-series models assess signal strength and detect anomalies relative to baseline expectations. These combined methodologies generate real-time alerts for potential safety issues.
Roche reports a measurable acceleration in adverse event detection times. Internal benchmarks show improved sensitivity and decreased false positive rates. Regulatory agencies noted enhanced reporting compliance and researchers can now respond to safety signals more promptly.
Roche’s deployment of an AI-driven pharmacovigilance system demonstrates how modern technology elevates drug safety management. By automating data ingestion and interpretation, they enhance patient protection and regulatory responsiveness.
Read our detail project at An Advanced AI-integrated Speaking Application: Mastering the art of communication | SmartDev
Innovative AI Solutions
Emerging artificial intelligence applications continue to reshape decision-making and operational processes within the pharmaceutical industry. The following innovations illustrate next-generation capabilities and evolving opportunities.
Solution 1: AI‑Enabled Regulatory and Document Assistants
Pharmaceutical regulatory documentation can be voluminous and time-consuming to compile. Compliance workflows suffer from inconsistency due to manual authoring and diverse stakeholder input. Delays and human errors in filings can result in opportunity costs and legal exposure.
Companies are deploying generative AI models as document assistants. These tools assist in drafting, summarizing, and formatting clinical protocols, regulatory submissions, and internal reports. Natural language understanding ensures coherence and consistency.
Generative pre-trained transformer models parse regulatory guidance and past filings to generate compliant text. Users interact with the assistant through prompts, allowing topic-specific drafting and iterative revisions. The system can also auto-detect inconsistencies and apply standardized language.
Pharmaceutical firms report 20 percent to 30 percent time savings in document preparation cycles. Teams benefit from enhanced consistency and reduced manual editing. Compliance review processes improve due to higher initial quality.
AI-powered document assistants bring greater efficiency and reliability to regulatory affairs. By combining advanced NLP with structured compliance protocols, they enable more streamlined documentation and improved stakeholder alignment.
Solution 2: In Silico Clinical Trial Modeling
Traditional clinical trials require extensive time and significant financial investment. Recruitment bottlenecks, trial dropout, and protocol amendment delays are frequent obstacles. Planning scenarios for varying patient cohorts is complex and resource-intensive.
Pharmaceutical innovators are embracing in silico clinical trial modeling. Through AI-driven simulations, organizations can design and refine trial protocols virtually before initiating live studies. Virtual cohorts and treatment scenarios are modeled in a controlled, scalable environment.
Generative models simulate physiological responses across synthetic patient populations. Predictive analytics estimate attrition rates, dosing effects, and safety signals. Multiple scenario runs help design adaptive trials with optimized endpoints and statistical power.
Early adopters demonstrate up to 40 percent reductions in protocol planning time. Trial designs optimized through simulation often show increased statistical confidence and reduced amendment rates. The virtual modeling stage lowers overall trial cost and risk profile.
In silico clinical trials exemplify how AI can de-risk and accelerate traditional trial pathways. By simulating complex biological and procedural dynamics, the pharmaceutical industry gains precision and scalability before committing to real-world execution.
Solution 3: Agentic AI for End‑to‑End Pipeline Orchestration
Pharmaceutical operations span many complex domains including discovery, manufacturing, and supply chain logistics. Process breaks and siloed data ecosystems frequently result in inefficiency. Human oversight is required at most steps, slowing decision cycles.
Agentic AI platforms function as autonomous orchestrators of pharmaceutical workflows. These systems can coordinate drug discovery pipelines, manage resource scheduling, and track patient analytics without constant supervision. They integrate cross-domain data and task triggers.
Agents use reinforcement learning and planning algorithms to initiate and sequence tasks. They adapt based on real-time feedback and evolve decision policies. Integration layers communicate with lab systems, manufacturing sensors, and logistics platforms.
Organizations that adopted agentic platforms report accelerated workflows and improved resource allocation. Manual touchpoints decrease by up to 50 percent, and operational bottlenecks are automatically resolved. The end‑to‑end orchestration improves both consistency and outcome predictability.
Agentic AI systems mark a transformative shift from isolated automation to unified, intelligent orchestration. By embedding autonomy and adaptivity, they support scalable, high-integrity pharmaceutical operations.
To explore more the effective of AI adoption, you can find information about Our projects and solutions we’ve developed in collaboration with our valued clients.
AI‑Driven Innovations Transforming Pharmaceutical R&D
-
Emerging Technologies in AI for Pharma
Generative AI is now a cornerstone of drug discovery: by generating molecular structures and predicting biological activity, it accelerates target identification and design. For example, AI-designed drugs from Exscientia entered clinical trials in record time—12 months instead of years. Insilico Medicine used generative adversarial networks and reinforcement learning to design novel compounds, progressing a pulmonary fibrosis candidate at one‑tenth the usual cost. Similarly, Pfizer’s collaboration with NVIDIA’s Ignition AI Accelerator and Saama expands AI-driven data analysis across R&D and regulatory workflows.
Computer vision and machine learning empower data‑driven decisions in trials and imaging. Janssen used interpretable AI to optimize site selection in vaccine trials, reducing trial time by 33 % and participant count by 25 %. In oncology, systems like Exscientia’s AI have personalized treatments for 143 patients with advanced blood cancers—54 % controlled cancer significantly longer than prior therapy.
-
AI’s Role in Sustainability Efforts
AI-driven predictive analytics optimize resource allocation and reduce waste. RELEX Solutions’ AI forecasting module delivered a 388 % ROI for AAH Pharmaceuticals—cutting inventory costs by $12.5 million over three years. McKinsey and PwC note that AI industrialized across production, supply chain, R&D, and commercial units could potentially double pharma operating profits, unlocking an estimated $254 billion globally by 2030.
Smart manufacturing, driven by real-time AI monitoring and process optimization, enhances sustainability by minimizing energy use and material waste, while ensuring regulatory compliance.
How to Implement AI in Pharmaceutical Industry
-
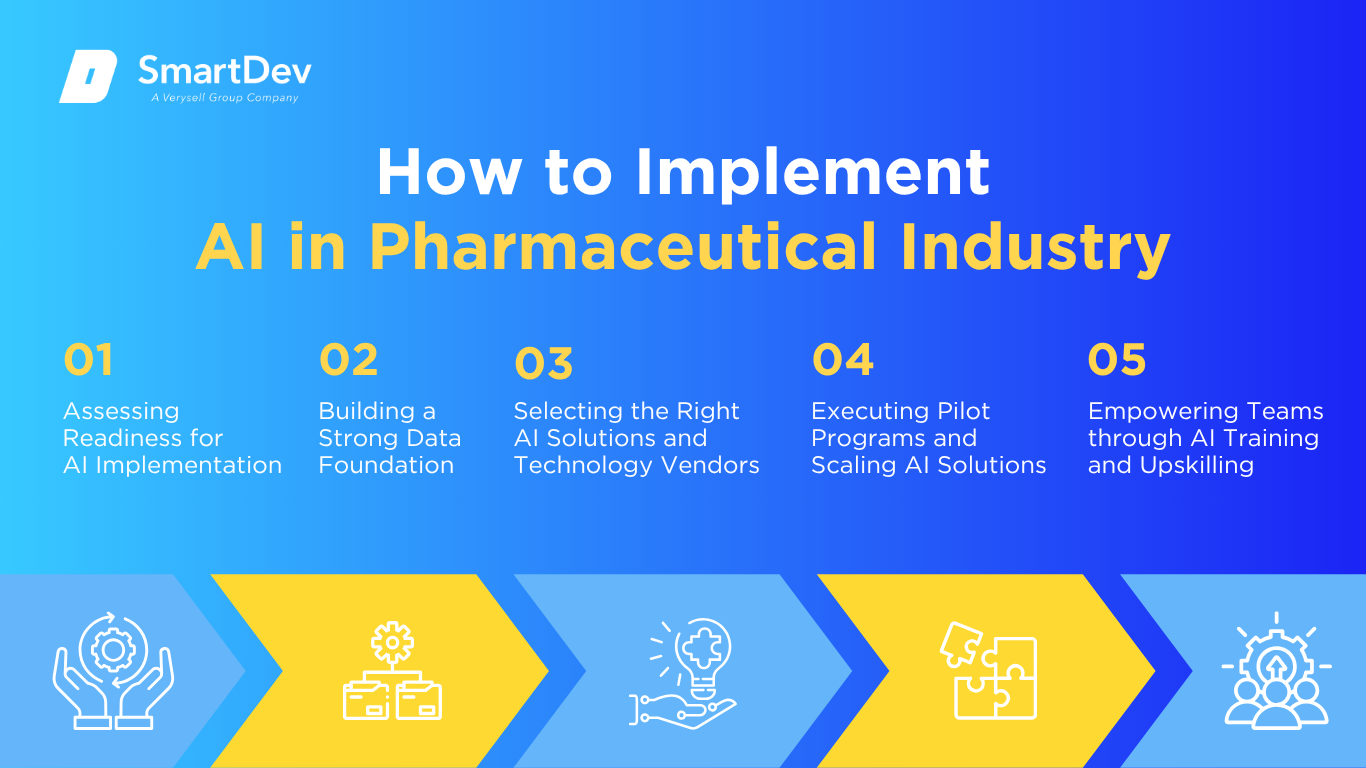
Assessing Readiness for AI Adoption
Begin by identifying high-impact areas suitable for AI integration, such as drug discovery and trial optimization. Evaluate your data infrastructure, available talent, and organizational appetite for innovation. Larger players like J&J and Merck have upskilled tens of thousands of employees in AI literacy—J&J trained 56,000 to use generative tools before allowing access. Smaller biotech firms, meanwhile, may start with pilot programs and external education before scaling.
-
Building a Strong Data Foundation
AI success rests on clean, well-curated data. Invest in robust data governance to unify and standardize datasets across clinical, genomic, and operational streams. This ensures machine learning models are trained accurately and effectively. Partnering with vendors that support secure EHR or biometrics data integration—from sources like Pfizer or Merck—can streamline data readiness and compliance.
-
Choosing the Right Tools and Vendors
Select AI platforms with proven pharma applications. Insilico and Exscientia have rapidly advanced AI-driven molecule design. Pfizer uses Saama for clinical data transformation, and AION Labs offers specialized ventures such as DenovAI for antibody discovery. Make vendor selection based on domain knowledge and regulatory readiness.
-
Pilot Testing and Scaling Up
Begin with targeted pilots—such as AI‑enabled site selection or supply forecasting—before enterprise-wide deployment. Track metrics like efficiency improvements and cost savings relative to investment. When results demonstrate value, scale gradually through additional use cases.
-
Training Teams for Successful Implementation
AI fluency is a must. Merck’s internal GPTeal platform supports over 50,000 users, with webcasts and boot camps for different roles. J&J supports immersive AI programs covering prompt engineering and summarization, which are critical for LLM adoption. Training initiatives help integrate AI seamlessly into daily workflows.
Measuring the ROI of AI in Pharma
-
Key Metrics to Track Success
Success metrics should include time-to-market reduction, cost-of-therapy development, yield improvements in R&D, and supply chain efficiencies. For example, clinical trials that adopt AI have reported cost savings as high as 70 % and durations surging by 80 %. Inventory optimization initiatives can be quantified by ROI and cost avoidance, as seen in supply chain AI implementations.
-
Case Studies Demonstrating ROI
RELEX Solutions’ deployment at AAH Pharmaceuticals achieved a staggering 388 % ROI within six months, cutting inventory carrying costs by $12.5 million over three years. Janssen’s AI site prediction model reduced trial time by 33 % and participant numbers by 25 % . Deloitte reports that leveraging AI pipelines can cut development costs by up to 70 %, as evidenced by Insilico’s cost-effective candidate INS018‑055.
Understanding ROI is possibly a challenge to many businesses and institutions as different in background, cost. So, if you need to dig deep about this problem, you can read AI Return on Investment (ROI): Unlocking the True Value of Artificial Intelligence for Your Business
-
Common Pitfalls and How to Avoid Them
Many firms overestimate data readiness or lack clear KPI frameworks. Low model adoption often follows poor user training or unclear business alignment. Avoid these by piloting use cases with measurable success, investing in data hygiene, forming cross-functional AI teams, and building human‑in‑the‑loop workflows that blend AI with domain expertise.
Future Trends of AI in Pharma

-
Predictions for the Next Decade
By 2030, AI will be embedded across pharmaceutical processes—from autonomous molecule design to real-time production optimization and AI-guided clinical decision support. Big-data–powered personalized medicine will tailor therapies at scale. McKinsey predicts billions in annual value from generative AI across the pharma ecosystem. Demand for AI-regulatory compliance, driven by frameworks like QA-RAG, will intensify.
-
How Businesses Can Stay Ahead of the Curve
To remain competitively agile, companies must industrialize AI by scaling successful pilots, forging partnerships with tech-focused AI startups, continuously upskilling staff, and building interoperable data systems. Leadership must foster a culture open to experimentation and anchored in metrics—just as early movers like Pfizer, Merck, J&J, and Insilico have done.
Conclusion
-
Summary of Key Takeaways on AI Use Cases in Pharma
AI accelerates drug discovery, optimizes clinical operations, enhances supply chain efficiency, and makes personalized medicine a reality. With cost savings of 70 % and ROI reaching nearly 400 %, AI is delivering tangible returns across the value chain.
-
Call-to-Action for Businesses Considering AI Adoption
If you’re a pharma leader, start with a clear pilot aligned to strategic goals, invest in robust data infrastructure, cultivate AI literacy, and partner with proven vendors. The opportunity is enormous—and missing this wave could cost you billions in both savings and innovation.
References
- https://litslink.com/blog/use-cases-of-ai-in-pharma-how-to-leverage-it
- https://www.mckinsey.com/industries/life-sciences/our-insights/generative-ai-in-the-pharmaceutical-industry-moving-from-hype-to-reality
- https://masterofcode.com/blog/generative-ai-chatbots-in-healthcare-and-pharma
- https://www.strategyand.pwc.com/de/en/industries/pharma-life-sciences/re-inventing-pharma-with-artificial-intelligence.html
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10385763/
- https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/ai-in-pharma-and-life-sciences.html
- https://www.maersk.com/insights/digitalisation/2024/06/18/artificial-intelligence-in-pharmaceutical-industry






